What's new in biology: October 2025
Safer transplants, new antibiotics, the rise of paper mills, long-term flu protection, slowing down ageing, and more.
Asimov Press’s Niko McCarty and our own Saloni Dattani review recent events in biotechnology and medicine.
A new way to hide transplanted cells from the immune system
Recipients of organ transplants are typically given lifelong immune suppression after the procedure to prevent their immune cells from attacking donor cells.
But this leaves recipients chronically vulnerable to infections, and so for a long time, researchers have looked for ways to avoid suppression. Methods have included encapsulating donor cells within membranes, using donor bone marrow to retrain the immune system, and delivering immune-modulating drugs only to the graft site.
These methods are good, but imprecise, as they rely on adjusting the immune system broadly. A new approach instead focuses on the transplant cells themselves: a 42-year-old man with type 1 diabetes has become the first person to receive a transplant with gene-edited cells that are less noticeable to the immune system.
In this case, the immune protection is built into the donor cells themselves, potentially allowing doctors to forgo the need to match donors entirely.
The scientists used CRISPR-Cas12b and a lentivirus vector to inactivate two genes in the pancreatic islet cells that were being transplanted. Inactivating the genes removed class I and class II HLA molecules, which are tags that immune cells use to recognise foreign tissue. The scientists also added a CD47 protein, which signals ‘don’t eat me’ to immune cells.
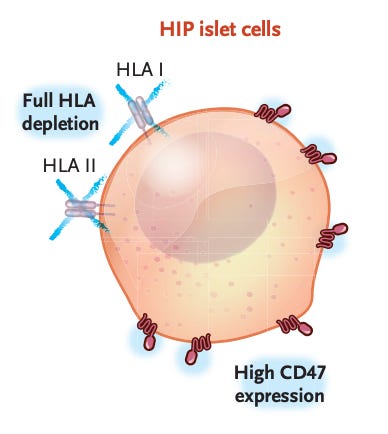
In the first 12 weeks after the transplant, the man’s immune system responded to unedited cells that remained in the graft, but showed no reaction to the fully edited cells. The edited cells survived and produced insulin, without causing any notable side effects.
In principle, the same strategy could be applied to many donor-derived cell therapies: from heart muscle cells for repairing damage after a heart attack, to neurons for Parkinson’s disease, to retinal cells for restoring vision.
AI tools create bespoke antibiotics
In the last few years, there have been many papers that use AI tools to either design or discover new antibiotic molecules. Perhaps most famously, in May 2023, researchers used a neural network to discover abaucin by testing 7,500 molecules against A. baumannii and then used those data to train the neural network to predict whether other molecules would also be effective against the microbe.
The abaucin paper proved that AI could find hidden antibiotics amongst existing chemicals; a new study by Aarti Krishnan and colleagues shows that AI can create entirely new antibiotics; chemicals that do not exist in nature.
The researchers began with an AI model that had previously been trained on ‘growth inhibition data for 38,765 compounds screened against’ two microbes. Next, they built a database of >45 million chemical fragments ‘with chemically meaningful structures’, and automatically compared them to a ‘manually compiled set of 559 known antibacterial compounds’. They did this step to make sure that their generated batch of 45 million chemical fragments would sample a wide swath of potential chemicals not covered by existing molecules.
Then, the authors screened this large fragment library using an AI model called Chemprop which predicts whether each molecule is likely to have antibiotic activity. More than 1 million molecules passed this filter. After filtering out molecules likely to be toxic, and doing some further computational checks, the researchers ultimately selected 80 compounds that were ‘structurally dissimilar from each other and had the highest predicted antibacterial scores’. Of those 80 compounds, two could be chemically synthesized with high purity. Those two molecules were called NG1 and NG2.
NG1, but not NG2, was then shown to inhibit growth of multidrug-resistant N. gonorrhoeae in the laboratory. This AI-designed antibiotic is structurally distinct from all known antibiotics and, according to the authors, likely acts through an entirely uncharacterized mechanism to kill microbes.
Paper mills are spreading
There is a large industry in science where companies known as ‘paper mills’ sell fake papers. For a fee, these firms produce bogus research results and offer them to scientists who need publications to get jobs, grants, or promotions. According to a new paper, there are now brokers, middlemen, editors, and even entire journals cooperating with these paper mills.
To figure this out, the study authors turned to an open-access journal, called PLoS One, which publicly discloses which editor accepted each published study. (This transparency is a good thing, and the journal was only used as a case study.) The study’s authors used statistical tests to check whether specific editors at PLOS One were likely to be working with paper mills.
Between 2006 and 2025, the journal relied on 18,329 editors to publish more than 276,000 articles, of which 702 were ultimately retracted. Using a Poisson binomial test, the study’s authors calculated ‘whether each editor accepted ultimately retracted...articles significantly more often than expected by chance alone.’ The answer was yes: 22 editors at PLoS One accepted articles ‘that were retracted significantly more frequently than one would expect by chance.’
These editors oversaw just 1.3 percent of all articles published in the journal, and yet those articles accounted for 30.2 percent of all retractions. The problems have been worsening, with the number of papers suspected to have been produced by paper mills doubling every 1.5 years even though total publications have only doubled every 15.
Clinical trials
A new long-acting drug could change how we prevent the flu. After a single injection lasting six months, Cidara’s experimental antiviral reduced flu by around 76 percent in a phase 2 trial of 5,000 adults – a performance comparable to the most effective flu vaccines. Antivirals with such long-lasting effects are becoming more common as researchers make progress on ways to release drugs more slowly within the body, and identify drugs that remain potent at very low doses. Cidara’s next trials will test whether combining the antiviral with vaccines could offer even stronger protection for vulnerable groups.
A new early-stage trial suggests that mRNA technology could be used to overcome some of the problems with HIV vaccines. Delivering the HIV’s envelope protein as a vaccine changes its shape. This exposes irrelevant regions the immune system can easily target, and distracts antibodies from the hidden, protective sites that matter most. The new mRNA vaccines avoid this problem by anchoring the envelope protein in the membranes of cells, preserving its natural shape. In 108 healthy volunteers, about 80 percent of those who received the membrane-bound versions made antibodies that blocked infection in lab tests, compared to only 4 percent with the soluble-protein version. The results are promising but still preliminary. The long-term outlook, however, is uncertain, as US government funding cuts to mRNA vaccine research will slow or stall progress in this area.
Lab science
Most people think of CRISPR proteins such as Cas9 as DNA editors, but a new paper shows it is relatively straightforward to reprogram these proteins to target RNA instead. Deleting a stretch of just 55 amino acids from IscB (the evolutionary ancestor of Cas9), for example, made the protein lose its ability to bind DNA and instead prefer interactions with RNA. The truncated protein could cut mRNAs and silence gene expression. The same strategy also worked on other Cas proteins, including Cas9, suggesting many DNA editors are perhaps more versatile – and could therefore be reprogrammed to fix more diseases – than once thought.
Progeria, a rare genetic disease, affects about 150 children worldwide. It is caused by a single nucleotide mutation in the genome and causes rapid aging. Fortunately, there has been major progress in treatment in just the last two decades. The first FDA approved drug, lonafarnib, extended life expectancy by 2.5 years. But a different approach, which relies on base-editing therapies that use a modified form of gene-editing tools to ‘swap’ out one nucleotide in DNA for another, has now cut toxic progerin protein levels by 90 percent and tripled survival in mice. Human trials are expected soon.
Scientists have reconstructed the first full genome of the 1918 pandemic flu virus. It came from an 18-year old man who died in Switzerland from the first wave of the pandemic and whose sample was preserved as a specimen at the University of Zurich’s Human Remains Collection. To reconstruct the viral genome, they developed a new method for sequencing ancient RNA that works well on tiny, degraded fragments (the only kind left in such old tissue samples). Remarkably, the virus already carried genetic changes linked to adaptation to humans, meaning that these mutations had arisen very early in the pandemic.






Great stuff! (Well, not the paper mills) Thanks for sharing.
Really cool stuff, thank you!