What’s new in biology: September 2025
Gene therapy, parasites, narcolepsy drugs, protein nanoparticles, the 3D structure of genomes, and more.
Asimov Press’s Niko McCarty and our own Saloni Dattani review recent events in biotechnology and medicine.
The second patient in the world to receive ‘prime editing’ gene therapy for rare immune disease
A 56-year-old man with chronic granulomatous disease (CGD), which causes repeated infections and chronic inflammation of the intestine, became the second person in history to be treated with prime editing, a gene-editing technique that can rewrite DNA with high precision. His form of CGD was caused by a missing ‘GT’ in the NCF1 gene, which prevents immune cells from producing an enzyme called NADPH oxidase.
Prime editing is part of the CRISPR toolkit, but it adds new features that make it more precise and versatile.
Standard CRISPR-Cas9 works like molecular scissors: it cuts through both strands of DNA at a target site, and the cell’s own repair machinery fills in the gap. The process can be unpredictable.
Prime editing, by contrast, makes only a single-strand cut and brings along its own repair kit (which includes a modified Cas9 protein to find the target, a guide RNA to position it, and an attached reverse transcriptase enzyme that writes in the new, correct DNA sequence directly from a built-in template). This lets scientists change DNA bases exactly where needed, without relying on the cell to guess how to fix the break.
This makes it ideal for CGD, which is caused by tiny but critical errors, such as the patient’s missing ‘GT’ in the NCF1 gene. In this case, doctors removed his bone marrow stem cells, used prime editing to insert the missing letters, and returned the corrected cells to his body. Just 15 days later, the edited cells had been engrafted, and his immune cells regained enzyme function. His intestinal inflammation resolved.
David Liu, who pioneered this gene editing technique, explains how it works in a video below.
However, Prime Medicine, the biotech company that developed the therapy, has announced it will not continue developing this particular therapy. For ultra-rare diseases like this, the number of potential patients is tiny, and manufacturing each dose is complex because it’s individualized. Prime Medicine is looking for external partners to carry it forward.
A high-resolution, 3D structure of the entire E. coli genome
Genomes are often taught in school as being one-dimensional objects. A big enzyme called DNA polymerase zips along the DNA strand and makes copies prior to cell division. Another enzyme, called RNA polymerase, converts DNA into RNA in preparation for protein synthesis. And so on.
In reality, however, genomes are not one-dimensional objects defined solely by the sequence of their letters. Their shape is quickly changing, both in time and in space.
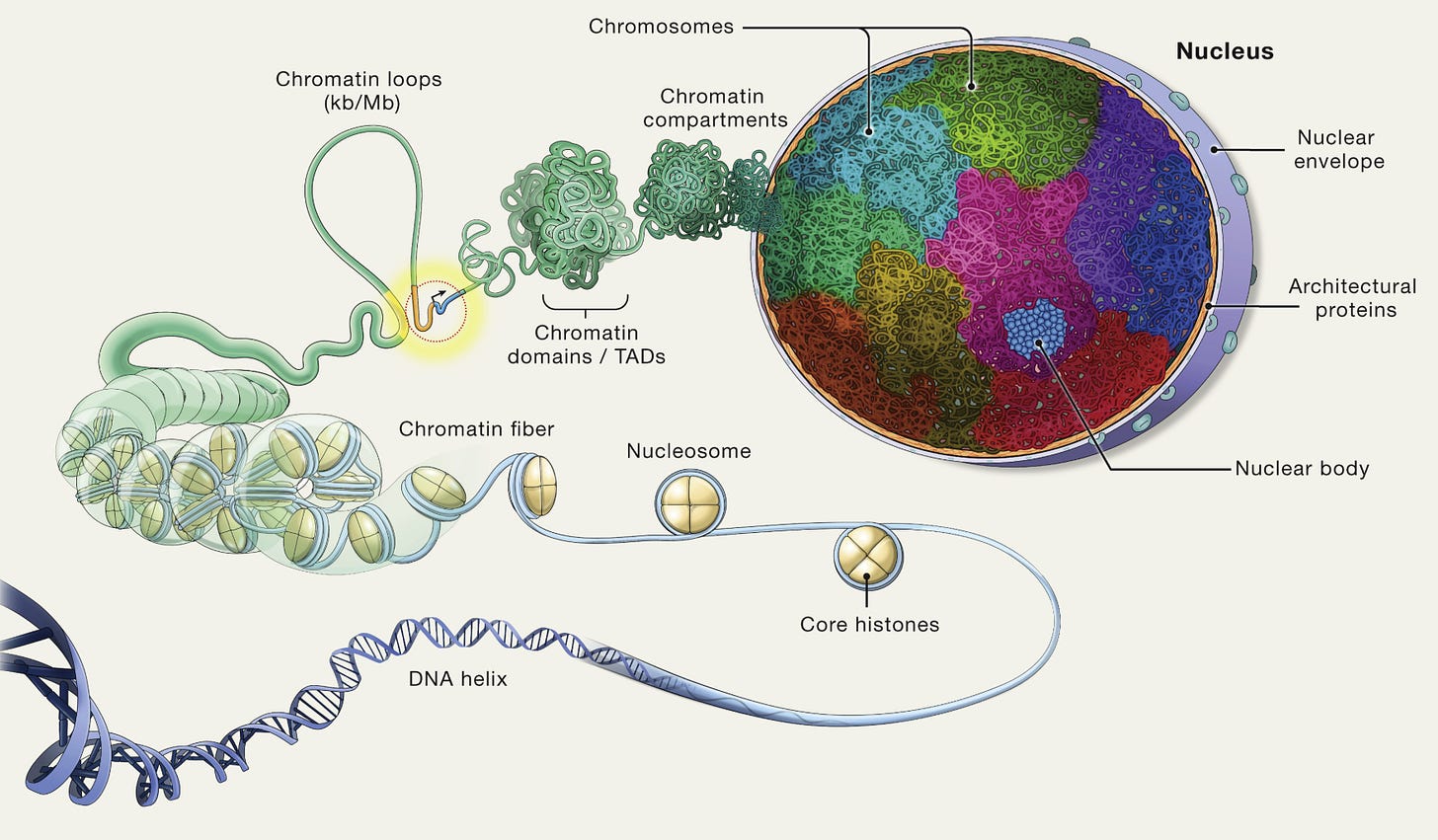
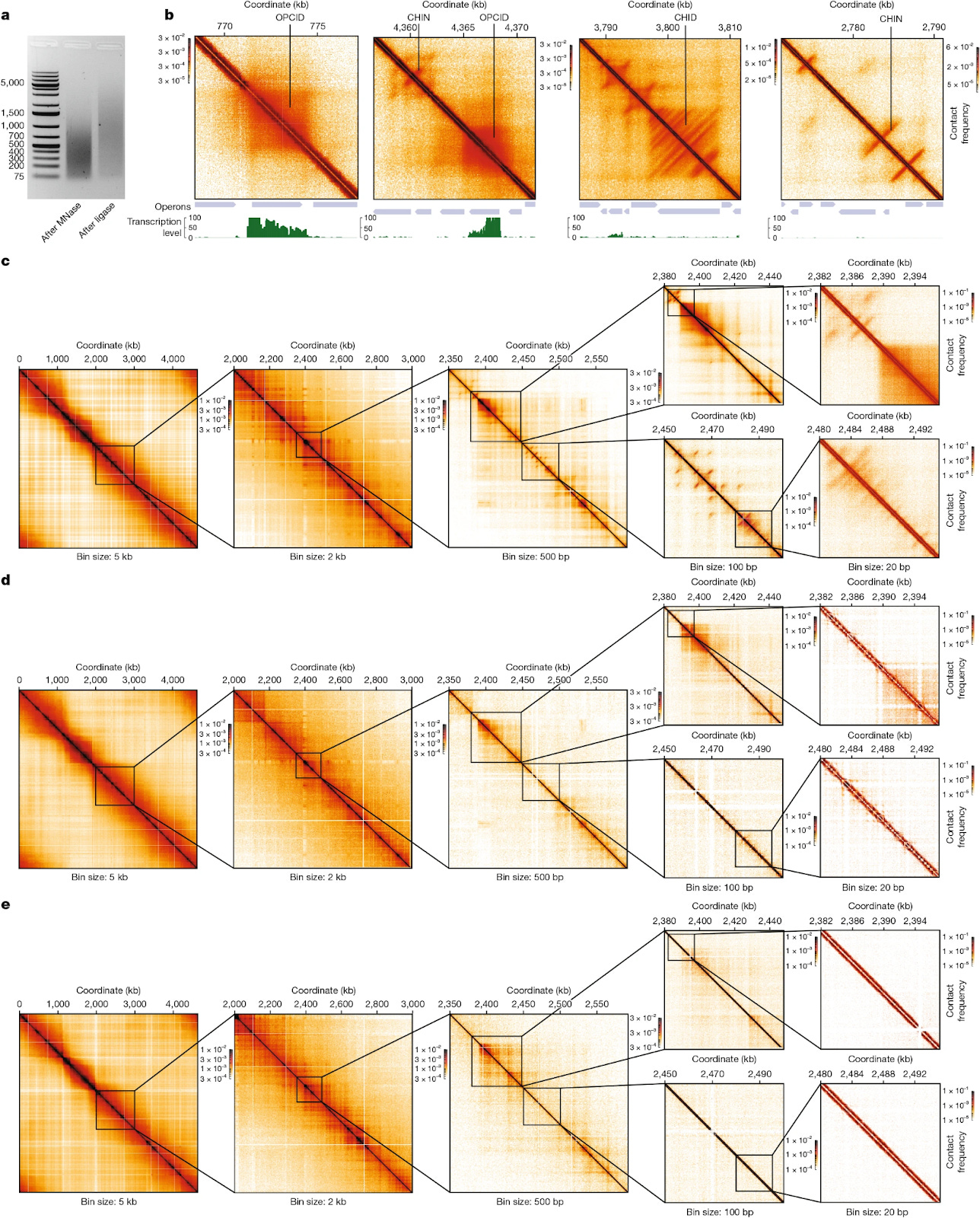
A new study in Nature reports this three-dimensional dynamism for the entire E. coli genome at a high resolution. With this map in-hand, researchers can clearly delineate which parts of the genome are physically close to each other in the cell, even though they may be far apart along their DNA sequence. One major finding is that clusters of related genes often fold into loops, bringing their start and end close together. This likely makes transcription more efficient, since enzymes can quickly loop back to the beginning instead of diffusing away.
The researchers created this map using a method called Micro-C. First, the cells were treated with a crosslinking agent that glued together any proteins and DNA that were physically touching in the cell. Next, researchers added an enzyme, called a nuclease, that chopped up the genome into tiny pieces. Last, an enzyme called DNA ligase was used to join tethered fragments together into a single molecule, which was then sequenced on a normal DNA sequencing machine. A computer algorithm then looked at which base pairs were joined together, and used that information to re-create a 3D map.
Clinical trials
A newly tested parasitic drug, moxidectin, looks like it could help tackle whipworm. Whipworm infects hundreds of millions of children in poorer rural areas, stunting growth and learning, and has little response from standard deworming pills, like albendazole. In a phase three trial in Tanzania, a single combination dose of moxidectin plus albendazole cured 69 percent of infections (meaning infected kids no longer had detectable worm eggs in their stool two to three weeks later) compared to just 16 percent with albendazole alone.
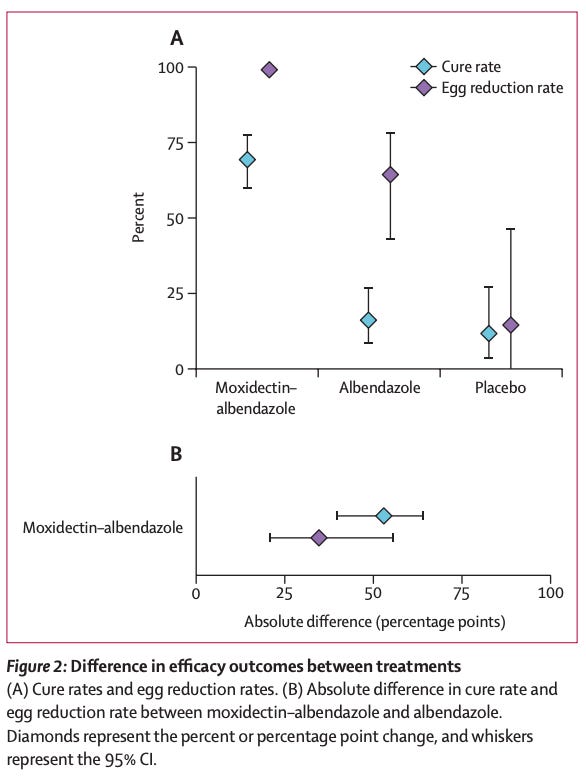
Results of the phase 3 trial of moxidectin and albendazole. Source: Annina Schnoz et al. (2025). Treating narcolepsy is hard: it is difficult to make a pill that can reach the brain, activate orexin receptors strongly enough to promote wakefulness, and still be safe. But a pharma company, Takeda, has reported that a new drug, ‘oveporexton’, has met its targets in phase 3 trials, making it likely to be approved by the FDA. In two studies involving 270 patients with narcolepsy type 1, subjects taking the drug had close to normal wakefulness in standardized lab tests, and reported less daytime sleepiness and improvements in their attention, daily functioning, and quality of life, compared to those on the placebo.
Lab science
Scientists have determined how antivirals could shut down the measles virus’s replication machinery. Measles still causes more than 10 million infections and over 100,000 deaths a year, mostly in unvaccinated children, and there are no approved antiviral drugs to help prevent or treat it. Using a technique called cryo-electron microscopy, they showed that a small molecule, named ERDRP-0519, latches onto a key control loop inside the virus’s polymerase enzyme, locking it into an ‘off’ position so it can’t copy RNA. The same drug also binds to the polymerase enzyme of Nipah virus, a highly fatal relative of measles. This suggests a single compound could target two of the most dangerous viruses in the Paramyxoviridae family.
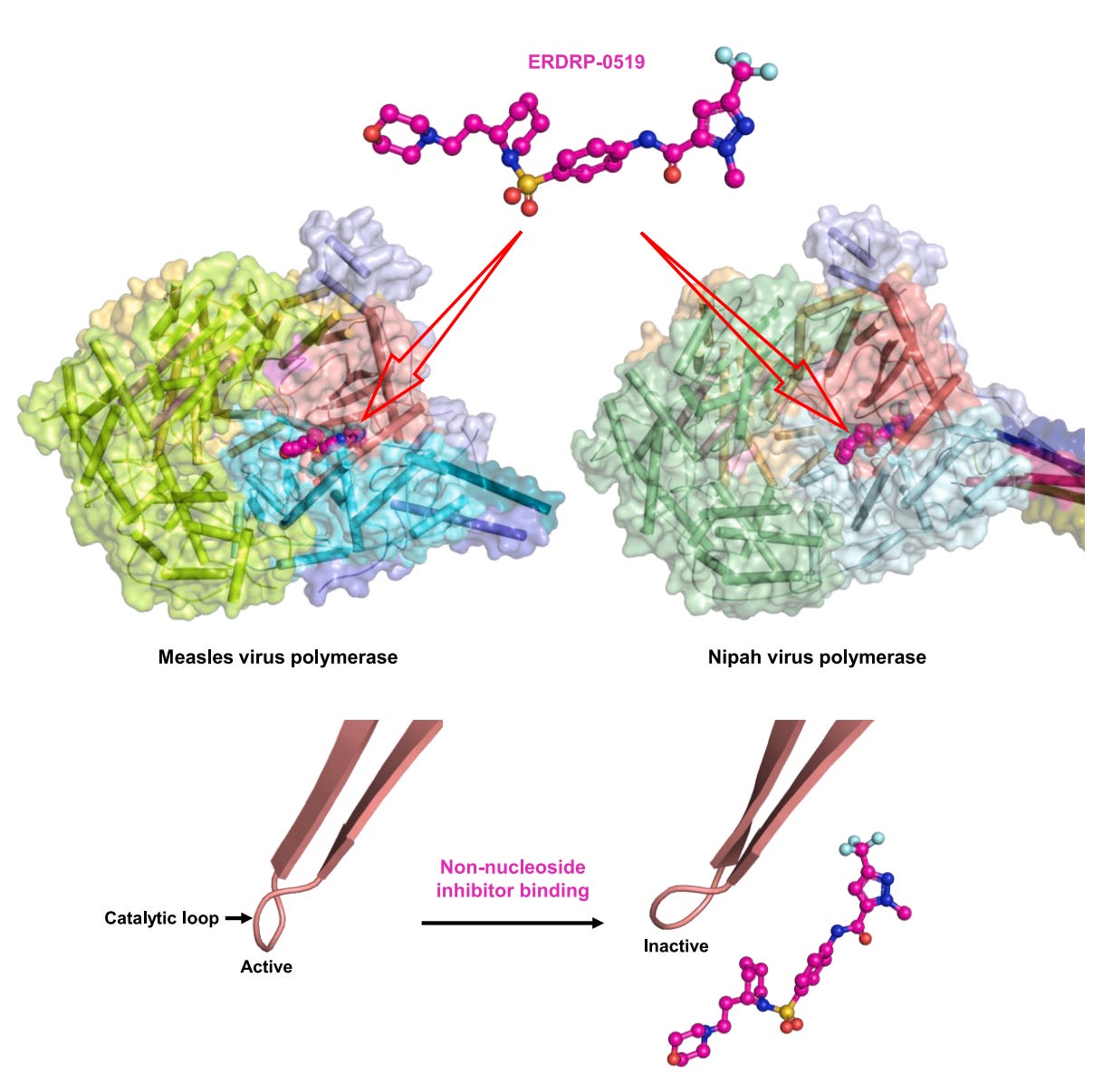
Structure of the polymerase enzyme in measles and Nipah virus, and how an inhibitor drug fit into the complex in laboratory research. Source: Yiru Wang et al. (2025)
Protein nanoparticles are tiny self-assembling structures that researchers often use as scaffolds to display vaccine antigens, hold enzymes together, or boost cell signaling. Historically, scientists have designed new types of protein nanoparticles which are symmetrical, meaning every side looks the same. Symmetry makes these nanoparticles easier to build, but it also limits what they can do. But now, researchers at the Institute for Protein Design have designed a new kind of protein nanoparticle with two distinct sides, meaning the same nanoparticle can interact with multiple different molecules at once. The researchers first built two halves of the nanoparticle, each with a slightly different set of subunits, and then joined them together. To demonstrate it worked, they engineered the particle’s structure so that each face displayed additional binding sites (‘minibinders’) pointing outward. The finished particles could then pull together two different types of receptor-coated beads. The approach could open the door to more versatile vaccines, diagnostics, and therapies.
It may now be possible to make CAR-T cells – a powerful form of immunotherapy used to treat cancer and certain autoimmune disease – directly inside the body using nanoparticles, instead of having to manufacture them in a lab. That matters because today’s CAR-T treatments, which reprogram a patient’s own T cells to hunt disease-causing cells, are life-saving but costly, slow, and out of reach for most patients. Working in mice and monkeys, scientists built lipid nanoparticles that carried mRNA instructions for a cancer-fighting receptor and targeted them to T cells. The treated animals rapidly generated their own CAR-T cells, which eliminated B cells. The next step would be to show that this translates to clearing B cell tumours or blocking the progression of autoimmune disease.
A new blog from OpenAI and Retro Biosciences describes a custom AI model, ‘GPT-4b micro’, that can design more efficient Yamanaka factors, or the proteins used to ‘reprogram’ mature cells back into stem cells. Shinya Yamanaka first discovered these proteins in 2006 by narrowing down 24 candidates down to just four essential ones: OCT4, SOX2, KLF4, and c-MYC. The new AI model (trained on an unreleased dataset incorporating protein sequences, biological text, and 3D structures) ‘designed’ new versions of SOX2 and KL4 that, when introduced into fibroblast cells, improved reprogramming efficiency by about 50-fold compared to natural forms of the proteins.
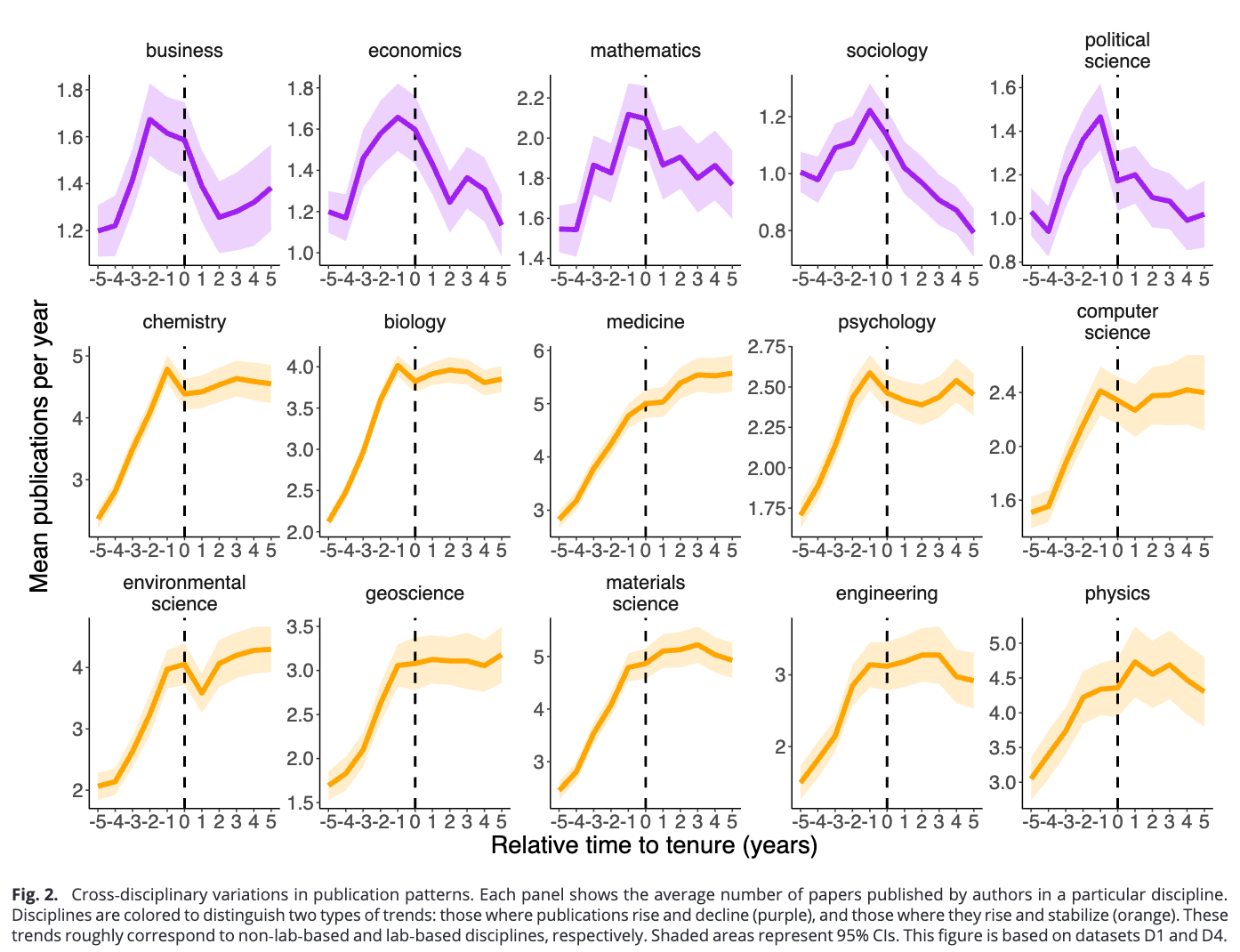
A large study, tracking the careers of more than 12,000 US faculty across 15 disciplines, measures just how much getting tenure (or a permanent faculty position) shapes research productivity. Publication rates rise steeply during the tenure track and peak just before tenure. In lab-based fields (like biology and chemistry), research output stays high afterwards and tenured faculty keep publishing at basically the same pace. But in non-lab fields like mathematics and sociology, productivity falls. After tenure, faculty are more likely to pursue novel or higher-risk projects, but their work garners fewer highly cited ‘hit’ papers.
What we enjoyed reading
AI will not lead to an Alzheimer’s cure. But will it help? In a mini series of blogposts, Jacob Trefethen describes ten bottlenecks in medical research, and explains compellingly where AI can make the most impact and why it won’t be enough for medical progress.
The FDA has a huge (yet inaccessible) archive of drug submissions. These files include thousands of pages covering trial design, manufacturing, safety, and regulatory negotiations. And many of the companies that submitted this paperwork have gone under! In a report for the Institute for Progress, Ruxandra Tesloianu explains how, by using bankruptcy law to acquire and publicly publish FDA filings from failed drug sponsors, the US could democratize regulatory know-how, and perhaps power AI systems that level FDA filings for smaller biotech companies.
An excellent post by Julia Rohrer about how correlations are more complicated than people think they are, how to interpret them properly, and how ‘things can get really messy once you calculate multiple correlations and compare them.’



