What's new in biology: summer 2025
The first gonorrhea vaccination program, contact lenses that see infrared light, the protein behind sweet tastes, a baby cured with gene therapy, and more
Asimov Press’s Niko McCarty and our own Saloni Dattani review more important things happening in the world of biotechnology and medicine.
Doctors made a custom CRISPR therapy to treat an infant with a rare genetic disease
A baby, named KJ, was born with a deficiency in carbamoyl phosphate synthetase 1 (CPS1). This deficiency means his liver couldn’t convert ammonia – which is made naturally when the body breaks down proteins – into urea, damaging his brain and liver. Babies born with CPS1 deficiency often end up staying in the hospital until they’re eligible for a liver transplant. They have to follow strict diets; specifically, a mix of natural protein and a medical formula that supplies only essential amino acids. If a baby’s protein intake is too high, their ammonia levels can spike, putting them at risk of permanent brain damage or death.
Researchers at the Children’s Hospital of Philadelphia diagnosed KJ within days of birth, and then spent about six months designing and testing a personalized gene-editing therapy to fix his deficiency. They used a CRISPR ‘base editor’ – a tool that rewrites a single DNA letter without breaking the DNA – delivered to his liver using lipid nanoparticles. KJ received the first dose in February this year. By April, he had received three in total.
Thanks to the therapy, KJ began tolerating more protein in his diet, and his blood ammonia levels dropped. He was able to reduce medications, gain weight, and move up on the growth chart. Because KJ’s condition was life threatening and rare, the team was able to administer the therapy under the FDA’s ‘single-patient expanded access’ pathway. Instead of going through the years-long clinical trial process, KJ’s treatment received FDA approval within weeks. This approach – sometimes called ‘N-of-1’ treatment – shows how regulatory systems can be flexible in urgent cases and might pave the way for faster development of personalized gene therapies in the future.


Arc Institute discovery allows longer gene edits
CRISPR tools have a shared limitation: they can only make small edits to genes, usually just a few letters long, which are helpful only for diseases caused by small, specific mutations, like KJ’s. In contrast, bridge recombinases, a new gene editing technology, are designed to work on a much larger scale. Like CRISPR, they use a programmable RNA molecule to find their targets, but instead of one RNA guide, they use a special ‘bridge RNA’ that folds into two loops. One loop binds to the target DNA, the other to the donor DNA, bridging them together. This allows the enzyme to rearrange large segments of DNA precisely.
In 2024, researchers at the Arc Institute discovered this mechanism and then engineered a version, called IS622, that works in human cells. They showed that it could make edits of nearly a million base pairs in length. The median human gene is around 24,000 base pairs in length, so a million base pair edit could affect the equivalent of around 40 genes. (The largest known human gene – dystrophin – is just over 2 million base pairs long.)
This means that bridge recombinases could allow scientists to edit much larger regions of the genome – including entire genes, long regulatory elements, or clustered gene families – which are out of reach for CRISPR-based tools.
New RSV vaccines are reducing infant hospitalizations in the US
Respiratory syncytial virus (RSV) is the leading cause of hospitalizations in infants in the US. Recently, however, scientists succeeded in developing two powerful tools for protection: a maternal vaccine that passes on protection to newborns and a protective antibody called ‘nirsevimab’, which is given directly to babies. Saloni previously wrote about RSV vaccines back in 2022. Since then, large phase three randomized trials have shown that each can reduce infant hospitalization rates by 80–90 percent. They were approved in mid-2023 and rolled out widely for the 2024–25 winter season.
Among the youngest infants (0–7 months) hospital admissions for RSV were around half the previous norm, as the top panel of the chart shows. Around half of infants this age received the vaccine or antibody. By contrast, hospitalizations for older toddlers (in the lower panels), most of whom didn’t receive the new products, stayed in line with or above past seasons. Widespread use of the new RSV vaccines and antibodies is already translating into fewer hospital beds filled with babies. Remember that only around half of the eligible infants received the vaccines that season. If vaccination rates were higher, the reduction in hospitalization rates would be even greater.

A new cholesterol drug
Lowering low-density lipoprotein (LDL) – the particle that carries cholesterol into artery walls – helps reduce the risk of heart attacks and strokes, and statins have helped millions do just that. But even the highest doses cannot reduce many high-risk people to the LDL targets that modern guidelines recommend.
Obicetrapib is a new drug that may help close that gap. It belongs to a drug class called CETP inhibitors, which block a protein that moves cholesterol between lipoproteins in the blood. Earlier CETP inhibitors had disappointing results due to side effects. But obicetrapib is designed to bind CETP more precisely, with fewer side effects.
Obicetrapib cut LDL cholesterol levels 30 percent in a phase three trial involving over 2,500 people with a history of heart disease or inherited high cholesterol. All were already on the maximum doses of statins and other lipid-lowering drugs, and with LDL levels above the target level. Importantly, the rate of side effects was similar between the two groups, suggesting the drug was tolerated well.

Silencing lipoprotein(a)
Another source of cardiovascular risk is lipoprotein(a), or Lp(a). Lipoprotein(a) is similar to LDL: both carry cholesterol through the blood. But Lp(a) has one important difference: it also carries an extra protein called apolipoprotein(a). That added piece makes it more harmful and harder for the body to clear. Unlike LDL, lipoprotein(a) levels are mostly influenced by genetics: around one in five adults have high lipoprotein(a) levels, but most don’t know it. A single gene, LPA, which produces apolipoprotein, confers most of the differences in risk between people.
Researchers are now developing ‘small interfering RNAs’, or siRNAs, to silence the LPA gene specifically. Unlike CRISPR and other gene-editing tools, which make permanent changes to DNA, siRNAs work by targeting the temporary messenger RNA (mRNA) instructions that cells use to make proteins. In many cases, siRNA effects are short-lived; in this case, the effect is unusually long-lasting – a single dose of siRNA can suppress the LPA gene’s activity for months. siRNAs are already approved for treating some rare liver diseases, and now several are being developed to target the LPA gene.
In a recent phase 2 trial, one such drug, lepodisiran, was given to 320 people with very high Lp(a). A single injection of the highest dose lowered their Lp(a) levels by 94 percent and kept them low for months – a dramatic reduction for any cholesterol-lowering therapy.

But while the trial showed that lepodisiran safely lowers Lp(a), it’s not clear yet how much that reduces heart attacks or strokes. Large studies find that cardiovascular risk increases in a continuous, log-linear form as Lp(a) levels rise, meaning people with lower levels are much healthier.
If further trials confirm the benefits, gene-silencing drugs could offer a new way to prevent cardiovascular disease.
The first gonorrhea vaccination program
The UK will become the first country in the world to offer a vaccine program against gonorrhea. Gonorrhea is a bacterial infection caused by Neisseria gonorrhoeae, which spreads through sex and can cause pain, discharge, inflammation, and sometimes infertility. Cases have been rising, and for every antibiotic class used against it, resistant strains have eventually followed.
N. gonorrhoeae is a challenging target: it varies its surface proteins, doesn’t generate lasting immunity after infection, and seems adept at dodging our immune system altogether. Fortunately, the MenB vaccine, developed to protect against meningitis B, which is caused by a close genetic relative, offers some ‘cross-protection’ against gonorrhea. Protection in trials so far has been moderate: one dose offered around 26 percent protection, while two doses boosted that to 33–40 percent.
Projections from Imperial College London suggest that if uptake is high, the program could prevent 100,000 infections and save the NHS nearly £8 million over the next decade.
A wearable device measures how much breast milk babies drink in real time
Right now, the way doctors and parents estimate how much milk a baby drinks during breastfeeding is surprisingly low-tech: they weigh the baby before and after a feed. This is often imprecise, especially for preterm infants. To solve this, researchers have developed a wearable device that uses four small electrodes placed on the skin to measure milk volume continuously and more accurately.
Breast milk has different electrical properties than fat, skin, or muscle, so when milk flows through the milk ducts, it changes how electricity moves through the breast. The device picks up on that shift. While impedance-based monitoring isn’t new, this version is portable, easy to use, and pairs with a smartphone. In a study of 12 mothers over 17 weeks, the device’s readings closely matched bottle measurements. Even without calibration, its software estimated milk volume within about three percent error.
Montana expands its Right-to-Try laws
Montana legislators passed a new law – called Senate Bill 535 – that expands access to experimental drugs that have passed through only Phase I clinical trials, even if those drugs remain under investigation for FDA approval. Montana already has a ‘Right-to-Try’ law from 2015, allowing terminally ill patients to try medications that had passed Phase I trials – similar to the federal Right-to-Try law passed in 2018. In 2023, Montana broadened its law, letting anyone access those therapies.
This latest bill, passed in May 2025, lets clinics in the state get licensed specifically as ‘experimental treatment centers,’ legally selling drugs and treatments right after their Phase I safety trials. They must also earmark two percent of their net profits each year to help residents more easily access those treatments. One downside of Montana’s approach, though, is that around 90 percent of all drugs that enter clinical trials fail, and most of those failures come at the later stages.
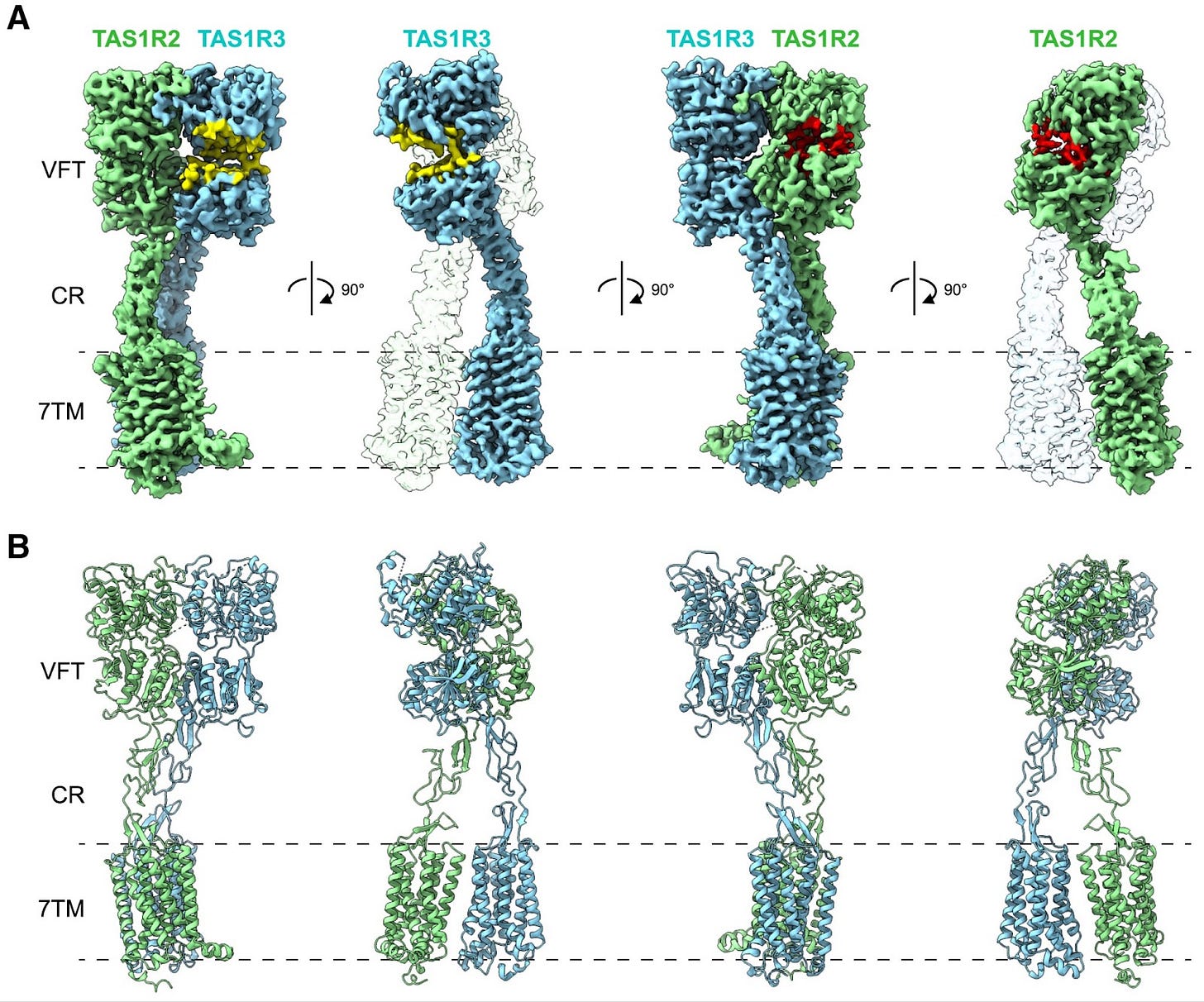
Scientists solve the structures of the ‘sweetness’ proteins
Humans have one main protein receptor that detects everything sweet, from glucose to sucrose and aspartame. Scientists have used cryo-EM imaging to solve the 3D structure of this receptor in tandem with various sweeteners. Cryo-EM works by quickly freezing proteins in their natural state, embedding them in a thin layer of ice. Electron beams are then shot at these samples multiple times, casting shadows to generate precise, composite 3D maps of each protein.
In this case, scientists captured the structure of the sweetness receptor while bound to two sweeteners: sucralose and aspartame. Both of these molecules fit neatly into the same ‘pocket’ within one half of the receptor, known as TAS1R2. The protein structures explain why some sweeteners, like aspartame, are far more potent than natural sugars. Aspartame’s chemical structure allows it to form tighter interactions within the TAS1R2 binding pocket; this molecular embrace stabilizes TAS1R2 in its ‘ON’ state much more strongly than sucrose.
Contact lenses to see infrared light
The cone cells in the human eye are sensitive to wavelengths from 400 to 700 nanometers. Infrared wavelengths are above that range, and thus humans cannot see them.
A group of researchers, writing in Cell, report that they’ve made contact lenses that convert infrared light into visible light, which can then be detected by the human eye. Each contact lens is packed with nanoparticles that absorb infrared photons at 808, 980, and 1,532 nanometers and then emit visible light. The lenses work without any neural interface or batteries, and supposedly cost about $200 to make. The images remain occasionally blurry and the lenses aren’t great at detecting more subtle infrared sources.
The competition behind AlphaFold is at risk of shutting down
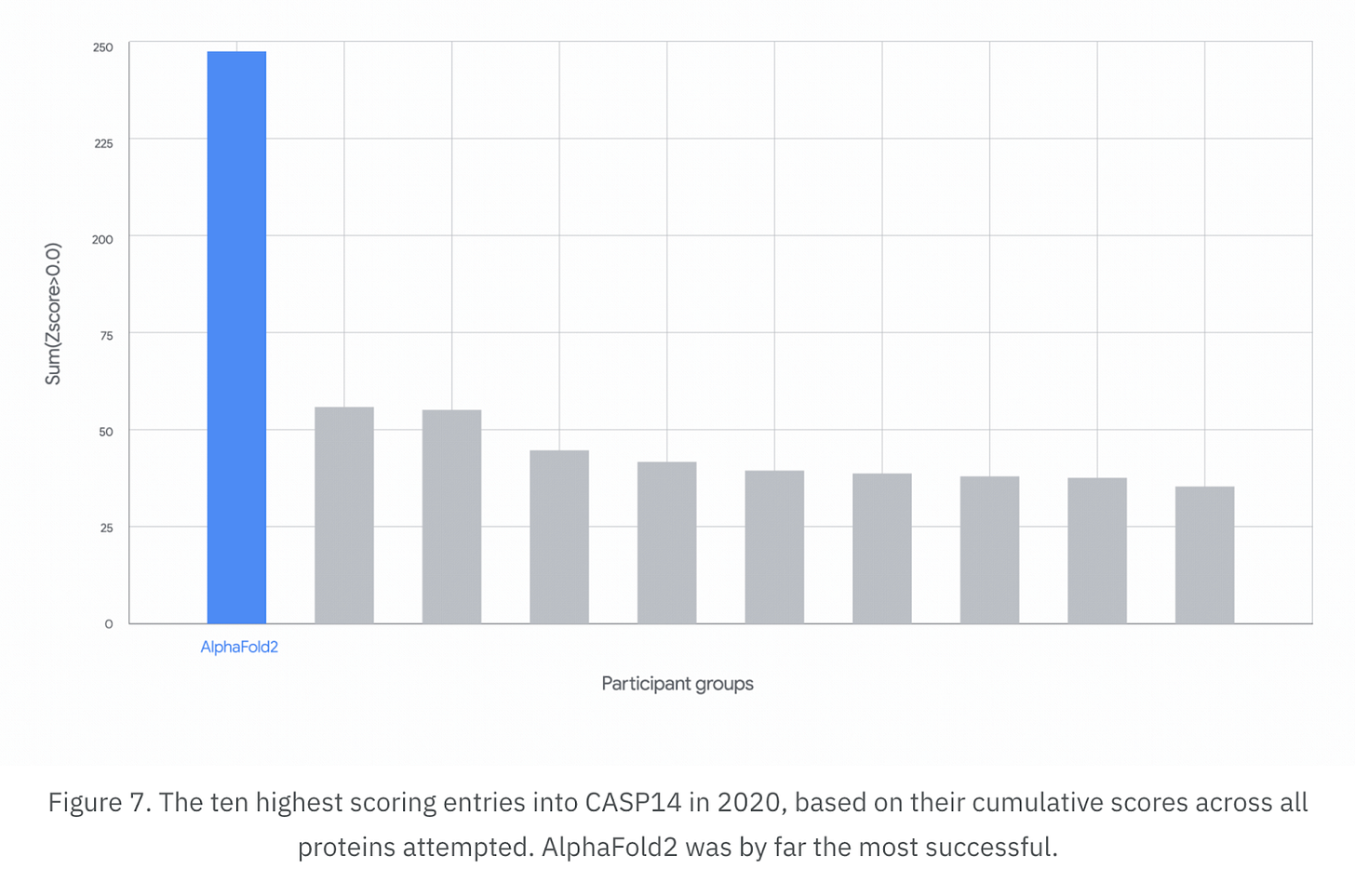
Critical Assessment of protein Structure Prediction (CASP) is a competition that began in 1994 and aimed to track progress against the ‘protein folding problem’ – the challenge of predicting the 3D shape of proteins based on their amino acid sequence. CASP’s goal was to create a standard and independent evaluation of computer models, allowing scientists to properly understand which models were performing better and where gaps remained.
It has been a huge success so far: in 2020, at CASP’s 14th competition, AlphaFold achieved a 90 percent success rate at predicting the structure of proteins, far beyond the roughly 50 percent achieved by other models. In 2024, it achieved a nearly 95 percent success rate.
But further objectives remain. Last year’s competition tested models’ ability to solve the structure of RNA molecules, interactions between proteins and molecules, and the shapes of disordered proteins. Unfortunately, funding from the NIH is now running out, as well as emergency support from UC Davis. Will the Trump administration reinstate funding, or will another funder step up?





I enjoy these posts. They are accessible, concise and uplifting as always.
Fantastic stuff. Thanks so much for sharing these stories.